Abstract
Introduction: Sickle cell disease (SCD) is caused by a single nucleotide mutation in the β-globin gene (HBB). CRISPR/Cas9 based gene correction approaches rely on inducing a double-strand break (DSB) at or near the sickle mutation, which subsequently results in gene correction via the homology-directed repair (HDR) or insertions/deletions (Indels) via non-homologous end joining (NHEJ). Frameshift Indels induce a premature stop codon and disrupt HBB production. Indels that preserve the reading frame may yield structurally abnormal β-globin chain with altered amino acid sequences and protein structures that can affect the globin chain stability, leading to thalassemia of uncertain pathogenic significance. The high rate and diversity of in-frame Indels as an unintended by-product of gene correction therapy confers a challenge for predicting therapeutic outcomes when it is not possible to frame the clinical behavior of hemoglobin (Hb) variants. Here we provided the molecular and structural characterization of the most commonly identified Hb variants due to CRISPR-induced in-frame deletions and proposed a putative pathogenic mechanism for the observed phenotype using human umbilical cord-derived erythroid progenitor cells (HUDEP2).
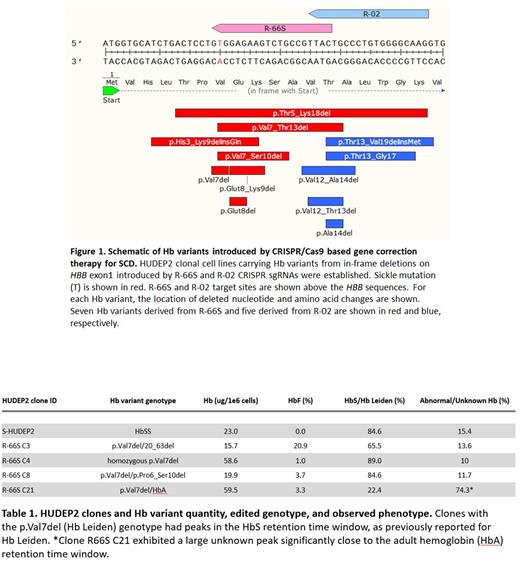
Methods: HUDEP2 clonal cell lines carrying mutated Hb variants from in-frame deletions on HBB exon1 introduced by R-66S and R-02 CRISPR sgRNAs currently in use in preclinical and clinical studies for correcting sickle mutation, respectively, were established, and clonal genotypes validated by next-generation sequencing and droplet digital PCR based copy number assay. The Hb variants derived from R-66S include p.Val7_Ser10del (-12bp), p.Val7del (-3bp), p.Glut8del (-3bp), p.Glut8_Lys9del (-6bp), pVal7Thr13del (-21bp), p.His3_Lys9delinsGln (-18bp), and p.Thr5_Lys18del (-42bp), in the order of allelic abundance observed in the bulk population of edited cells. The Hb variants derived from R-02 include p.Val12_Ala14del (-9bp), p.Val12_Thr13del (-6bp), p.Ala14del (-3bp), p.Thr13_Gly17del (-15bp), and p.Thr13_Val19delinsMet (-18bp). Selected ten clonal cell lines carrying a unique combination of genotypes and the parent HUDEP2 were cultured for expansion in serum-free media for one week. All clones were subsequently subjected to erythroid differentiation media for ten days. Samples were collected on days 4, 7, and 10 of differentiation for analysis via high-performance liquid chromatography (HPLC) using an ionic column to access the Hb profile and spectrophotometry via a Hb assay kit to assess the total Hb quantity.
Results: HPLC analysis demonstrated a significant qualitative variation in Hb profile in HUDEP2 clones carrying different Hb variants. p.Val7del is Hb Leiden, and the rest of Hb variants are not listed in the HbVar database. In clones carrying Hb Leiden in homozygous or heterozygous states, Hb Leiden eluted in the position of sickle Hb (HbS). A low percentage of multiple abnormal Hb X fractions was observed in all clones carrying Hb variants compared to parent HUDEP2, which hinted at the presence of unstable Hb variants or alpha precipitates. Levels of fetal Hb (HbF) increased in mutant clones, suggesting globin chain imbalance and hematopoietic stress. Clones missing multiple amino acids showed ineffective erythropoiesis and cell death by day 4 of differentiation, most likely exacerbated by the relative excess of the α-chains.
Discussion: We found that unintended gene modifications can significantly impact Hb quality and quantity, reinforcing concerns about the safety of gene-editing therapy for SCD. In patients, cells carrying in-frame deletions in the β-globin chain could produce an unstable Hb and may contribute to a β-thalassemia-like phenotype. More work is required to further assess the consequences of the diverse Hb variants in combination with other unintended modifications that disrupt HBB in a bulk population of gene-edited cells. Detecting, profiling, and reducing these unintended modifications remains a priority to increase the efficacy and safety of gene-editing therapy for treating SCD or β-thalassemia.
Disclosures
Hernandez:Global Blood Therapeutics: Research Funding. Sheehan:Global Blood Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal